
Attraction, that intangible force guiding human connections, is not solely the realm of emotions but a profound interplay of biophysics and chemistry. In this exploration, we delve into the intricate world where biology meets physics and chemistry, unraveling the mechanisms behind the captivating dance of attraction.
1. Molecular Forces in Attraction:
Attraction at the molecular level involves fundamental forces that govern interactions between biological entities. One key player is the van der Waals force, an attractive force arising from temporary fluctuations in electron distribution.
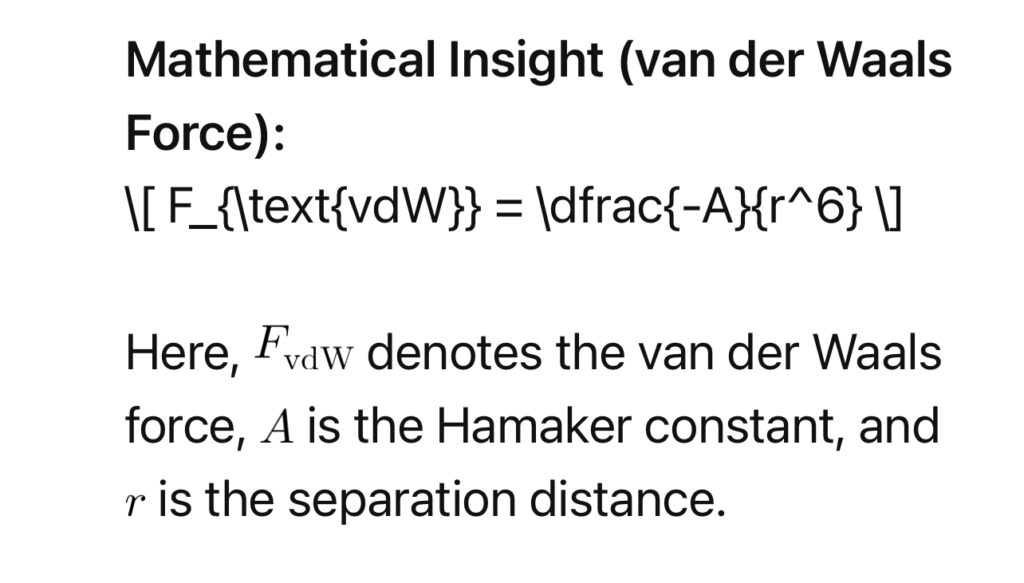
2. Electrostatic Interactions:
Charged particles, such as ions or polar molecules, contribute to attraction through electrostatic interactions. Coulomb’s Law provides insight into the force between charged entities.

3. Hydrogen Bonding:
Hydrogen bonding, a crucial player in biological attraction, occurs when a hydrogen atom is covalently bonded to a highly electronegative atom and is attracted to another electronegative atom.
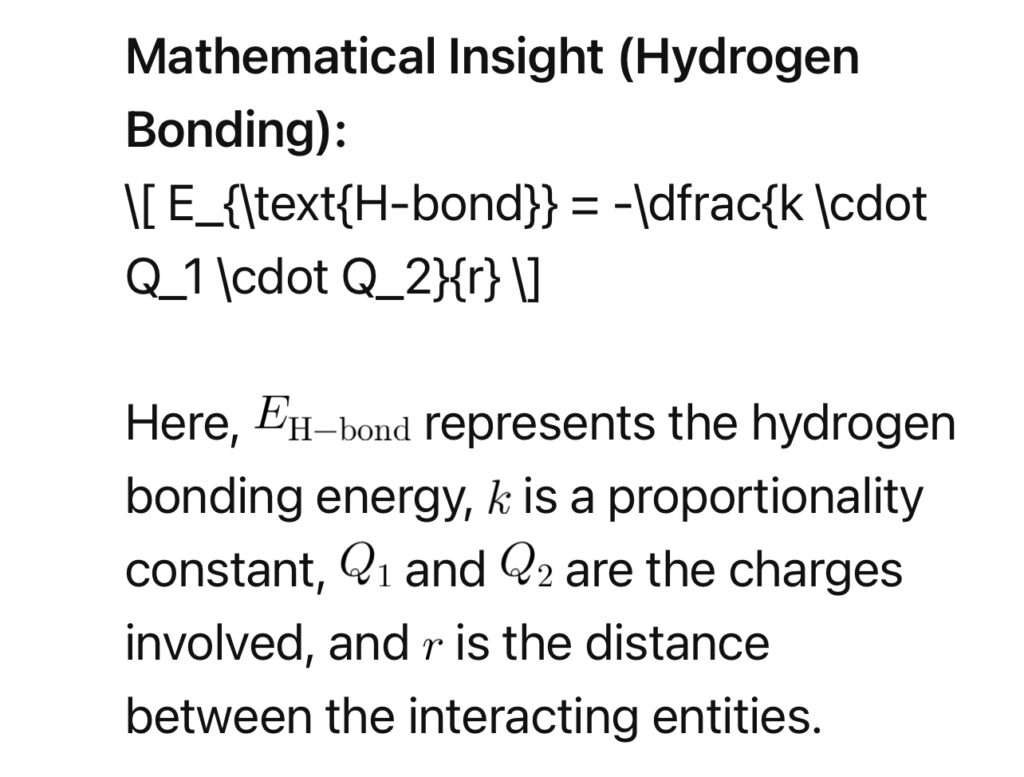
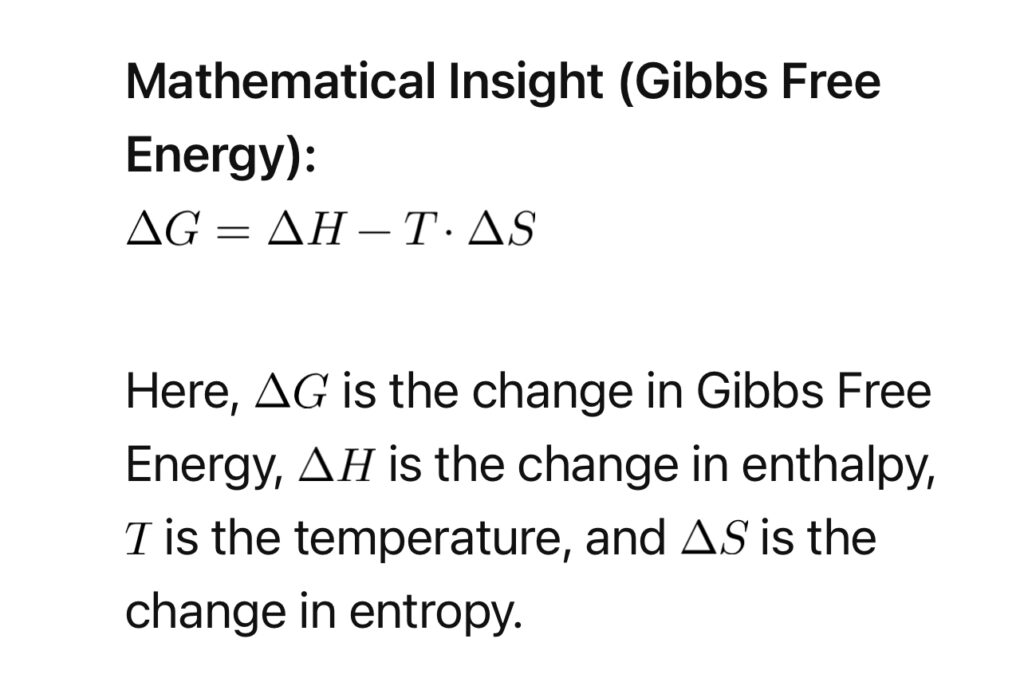
4. Thermodynamics of Attraction:
Thermodynamic principles shed light on the spontaneity and feasibility of attraction. Gibbs Free Energy (\(G\)) plays a crucial role, determining whether a process is energetically favorable.
5. Neurotransmitters and Chemistry:
Biological attraction is intricately tied to the release of neurotransmitters like dopamine and serotonin. These chemical messengers modulate mood and emotion, influencing the perception of attraction.

In conclusion, the biophysics of attraction emerges as a symphony of molecular forces, electrostatic interactions, hydrogen bonding, thermodynamic principles, and the intricate chemistry of neurotransmitters. As we decode these equations and delve into the mathematical underpinnings, the dance of attraction reveals itself as a beautifully orchestrated interplay of biological and physical forces.
